Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design and Ethics
2.3. Study Population
2.4. Clinical Data Collection
2.5. Follow-Up CT Scan
2.6. Statistical Analyses
3. Results
3.1. Study Population
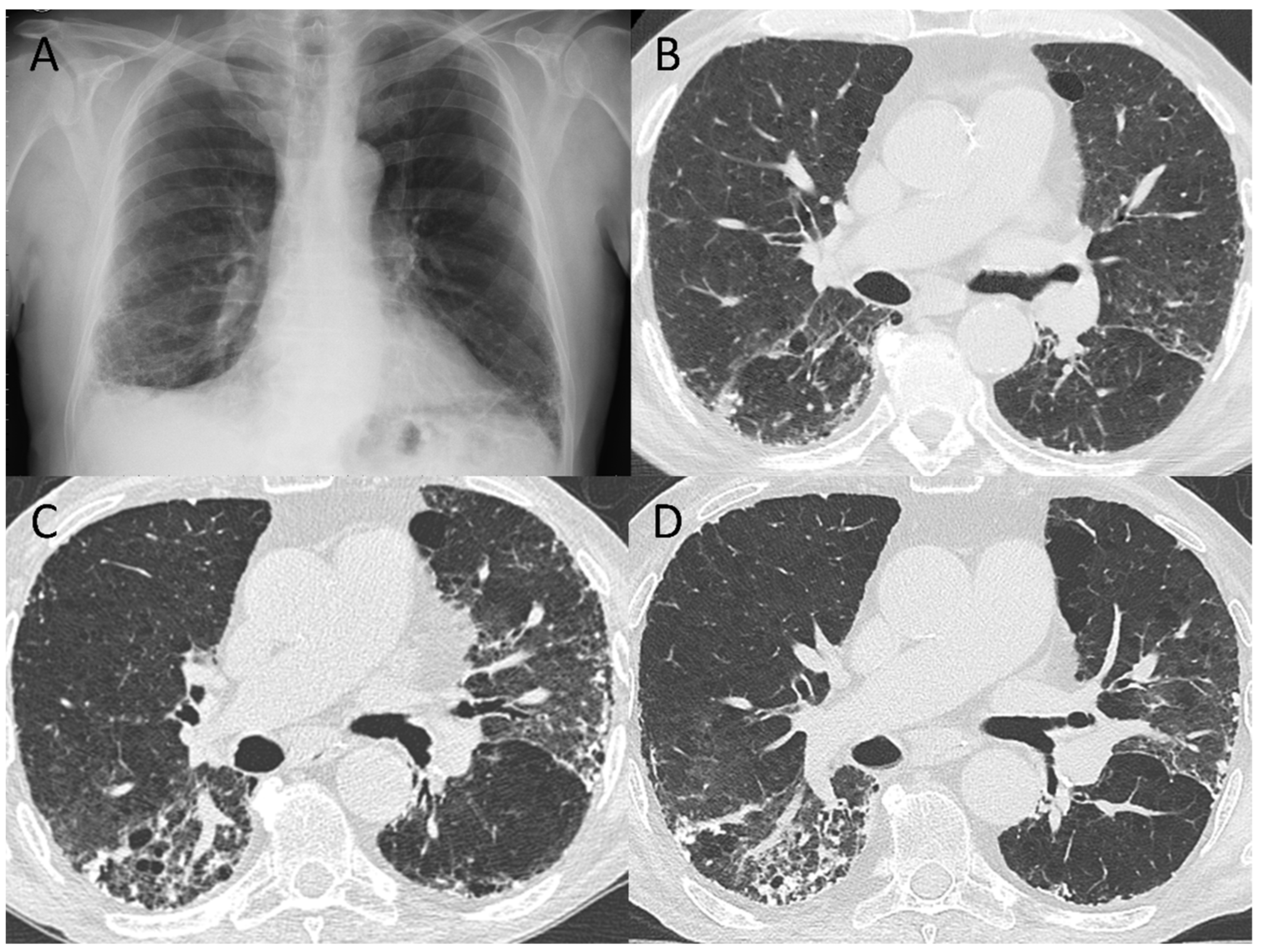
3.2. 5–7-Month Follow-Up CT Scan
3.3. Follow Up CT Findings: Comparison between the Two Cohorts
3.4. Pattern Evolution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Methods-CT Scan Technical Data
Appendix A.1. Reggio Emilia
Appendix A.2. Parma
Appendix B. Inter-Rater Agreement
| Reader 1-Reader 2 | Reader 2-Reader 3 | Reader 1-Reader 3 | ||||
|---|---|---|---|---|---|---|
| Kappa | p | Kappa | p | Kappa | p | |
| CT pattern (resolution/residual non-fibrotic/residual fibrotic) | 0.93 | <0.0001 | 0.90 | <0.0001 | 0.90 | <0.0001 |
| Global extension | 0.98 | <0.0001 | 0.98 | <0.0001 | 0.96 | <0.0001 |
| Overt GGO | 0.67 | <0.0001 | 0.86 | <0.0001 | 0.68 | <0.0001 |
| Barely visible GGO | 0.98 | <0.0001 | 0.97 | <0.0001 | 0.97 | <0.0001 |
| Number of lobes involved by GGO | 0.88 | <0.0001 | 0.93 | <0.0001 | 0.87 | <0.0001 |
| Parenchymal bands | 0.61 | <0.0001 | 0.72 | <0.0001 | 0.66 | <0.0001 |
| Consolidations | 0.75 | <0.0001 | 0.86 | <0.0001 | 0.89 | <0.0001 |
| Perilobular opacities | 0.72 | <0.0001 | 0.86 | <0.0001 | 0.86 | <0.0001 |
| Bronchiectasis | 0.67 | <0.0001 | 0.85 | <0.0001 | 0.72 | <0.0001 |
| Pattern of non-fibrotic abnormalities | 0.99 | <0.0001 | 0.98 | <0.0001 | 0.97 | <0.0001 |
| Subpleural reticulation | 0.68 | <0.0001 | 0.83 | <0.0001 | 0.74 | <0.0001 |
| Honeycombing | 0.40 | <0.0001 | 1.00 | <0.0001 | 0.40 | <0.0001 |
| Volume loss | 0.50 | <0.0001 | 0.79 | <0.0001 | 0.51 | <0.0001 |
| Cicatricial emphysema | 1.00 | <0.0001 | 1.00 | <0.0001 | 1.00 | <0.0001 |
References
- WHO. World Health Statistics. 2021. Available online: https://www.who.int/data/gho/publications/world-health-statistics (accessed on 30 January 2021).
- Hui, D.S.; Joynt, G.M.; Wong, K.T.; Gomersall, C.D.; Li, T.S.; Antonio, G.; Sung, J.J.Y. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005, 60, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.S.; Wong, K.T.; Ko, F.W.; Tam, L.S.; Chan, D.P.; Woo, J.; Sung, J.J. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005, 128, 2247–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.-W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.B.; Jun, K.I.; Kim, G.; Choi, J.-P.; Rhee, J.-Y.; Cheon, S.; Shin, H.S. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J. Korean Med. Sci. 2018, 33, e169. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Stylemans, D.; Smet, J.; Hanon, S.; Schuermans, D.; Ilsen, B.; Vandemeulebroucke, J.; Vanderhelst, E.; Verbanck, S. Evolution of lung function and chest CT 6 months after COVID-19 pneumonia: Real-life data from a Belgian University Hospital. Respir. Med. 2021, 182, 106421. [Google Scholar] [CrossRef]
- Wu, Q.; Zhong, L.; Li, H.; Guo, J.; Li, Y.; Hou, X.; Yang, F.; Xie, Y.; Li, L.; Xing, Z. A Follow-Up Study of Lung Function and Chest Computed Tomography at 6 Months after Discharge in Patients with Coronavirus Disease 2019. Can. Respir. J. 2021, 2021, 6692409. [Google Scholar] [CrossRef]
- Liu, M.; Lv, F.; Huang, Y.; Xiao, K. Follow-Up Study of the Chest CT Characteristics of COVID-19 Survivors Seven Months After Recovery. Front. Med. 2021, 8, 636298. [Google Scholar] [CrossRef]
- Caruso, D.; Guido, G.; Zerunian, M.; Polidori, T.; Lucertini, E.; Pucciarelli, F.; Polici, M.; Rucci, C.; Bracci, B.; Nicolai, M.; et al. Post-Acute Sequelae of COVID-19 Pneumonia: Six-month Chest CT Follow-up. Radiology 2021, 301, E396–E405. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, W.; Yue, J.; Qin, L.; Zhang, C.; Xu, S.; Liu, X.; Ni, W.; Xie, M. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci. Rep. 2021, 11, 13854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ding, C.; Yu, L.; Guo, W.; Feng, X.; Yu, L.; Su, J.; Xu, T.; Ren, C.; Shi, D.; et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Tonkin, J.; Devaraj, A.; Philip, K.E.J.; Orton, C.M.; Desai, S.R.; Shah, P.L. CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge. Radiology 2021, 5, 211746. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Yang, L.; Liang, B.; Ye, T.; Li, L.; Li, L.; Liu, D.; Wang, J.; Hesketh, R.L.; Zheng, C. Chest CT Patterns from Diagnosis to 1 Year of Follow-up in COVID-19. Radiology 2021, 5, 211199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, C.; An, X.; Xiong, Y.; Shang, Y.; He, J.; Qiu, Y.; Zhang, N.; Huang, L.; Jia, J.; et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int. J. Infect. Dis. 2021, 112, 173–182. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Zhang, X.; Jia, X.; Zheng, Y.; Shi, H. Fibrotic Interstitial Lung Abnormalities at 1-year Follow-up CT after Severe COVID-19. Radiology 2021, 301, E438–E440. [Google Scholar] [CrossRef]
- Martini, K.; Larici, A.R.; Revel, M.P.; Ghaye, B.; Sverzellati, N.; Parkar, A.P.; Snoeckx, A.; Screaton, N.; Biederer, J.; Prosch, H.; et al. European Society of Thoracic Imaging (ESTI), the European Society of Radiology (ESR). COVID-19 pneumonia imaging follow-up: When and how? A proposition from ESTI and ESR. Eur. Radiol. 2021, 29, 11. [Google Scholar] [CrossRef]
- Wells, A.U.; Devaraj, A.; Desai, S.R. Interstitial Lung Disease after COVID-19 Infection: A Catalog of Uncertainties. Radiology 2021, 299, E216–E218. [Google Scholar] [CrossRef]
- Wells, A.U.; Devaraj, A. Residual Lung Disease at Six-month Follow-up CT after COVID-19: Clinical Significance Is a Key Issue. Radiology 2021, 301, E406–E408. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Wells, A.U. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet. Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King TEJr Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; Behr, J.; et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Gosangi, B.; Rubinowitz, A.N.; Irugu, D.; Gange, C.; Bader, A.; Cortopassi, I. COVID-19 ARDS: A review of imaging features and overview of mechanical ventilation and its complications. Emerg. Radiol. 2022, 29, 23–34. [Google Scholar] [CrossRef]
- Ravaglia, C.; Doglioni, C.; Chilosi, M.; Piciucchi, S.; Dubini, A.; Rossi, G.; Pedica, F.; Puglisi, S.; Donati, L.; Tomassetti, S.; et al. Clinical, radiological, and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection [published online ahead of print, 2022 Mar 17]. Eur. Respir. J. 2022, 2102411. [Google Scholar] [CrossRef]
- Wu, X.; Dong, D.; Ma, D. Thin-Section Computed Tomography Manifestations During Convalescence and Long-Term Follow-Up of Patients with Severe Acute Respiratory Syndrome (SARS). Med. Sci. Monit. 2016, 22, 2793–2799. [Google Scholar] [CrossRef] [Green Version]
- Poerio, A.; Carlicchi, E.; Lotrecchiano, L.; Praticò, C.; Mistè, G.; Scavello, S.; Morsiani, M.; Zompatori, M.; Ferrari, R. Evolution of COVID-19 Pulmonary Fibrosis-Like Residual Changes Over Time-Longitudinal Chest CT up to 9 Months After Disease Onset: A Single-Center Case Series. SN Compr. Clin. Med. 2022, 4, 57. [Google Scholar] [CrossRef]



| Reggio Emilia Cohort Patients (n = 234) | Parma Cohort Patients (n = 171) | |
|---|---|---|
| Female Sex, n (%) | 70 (30%) | 56 (33%) |
| Age, mean ± SD (years) | 65.5 ± 11.1 | 63.6 ± 12.2 |
| Comorbidities (at least one), n (%) | 152 (65%) | 120 (70%) |
| Initial Symptoms | ||
| Fever | 192 (82%) | 118 (69%) |
| Cough | 134 (57%) | 68 (40%) |
| Dyspnea | 63 (27%) | 64 (37%) |
| Fatigue | 15 (6%) | 11 (6%) |
| Other Symptoms | 60 (26%) | 25 (15%) |
| Missing information | 13 (6%) | 37 (22%) |
| Laboratory and CT data at admission | ||
| SatO2, mean (±SD) (%) | 92.5 ± 4.7 | 91.6 ± 6 |
| C-reactive protein (CRP), mean ±SD (mg/dL) | 10.3 ± 7.6 | 13.6 ±7.3 |
| PO2, mean ± SD (mmHg) | 65.9 ± 14 | 75.1 ± 31 |
| White blood cells count, mean ± SD (109/L) | 6.8 ± 5.3 | 7.6 ± 4 |
| Lymphocytes percentage, mean ± SD (%) | 20.5 ± 36.3 | 28.7 ± 20 |
| CT disease extension at admission, median (IQR) (%) | 50 (30; 55) | 30 (15; 50) |
| Disease severity | ||
| Non-severe, n (%) | 79 (34%) | 63 (37%) |
| Severe, n (%) | 155 (66%) | 108 (63%) |
| Whole Population (n = 405) | Reggio Emilia Cohort (n = 234) | Parma Cohort (n = 171) | |||||
|---|---|---|---|---|---|---|---|
| Resolution | 225 (55.6%) | 126 (53.8%) | 99 (57.9%) | ||||
| Residual non-fibrotic abnormalities | 152 (37.5%) | 91 (38.9%) | 61 (35.7%) | ||||
| Global extension (%), median (IQR) | 20% (10%; 30%) | 20% (15%; 30%) | 15% (5%; 30%) | ||||
| Overt GGO | 20 (4.9%) | 20 (8.5%) | - | ||||
| Barely visible GGO | 110 (27.2%) | 69 (29.5%) | 41 (24.0%) | ||||
| Number of lobes involved by GGO, median (IQR) | 4 (3; 5) | 4 (3; 5) | 3 (2; 5) | ||||
| Parenchymal bands | 11 (2.7%) | 7 (3.0%) | 4 (2.3%) | ||||
| Lobar | - | - | - | ||||
| Peripheral | 11 (2.7%) | 7 (3.0%) | 4 (2.3%) | ||||
| Consolidations | 4 (1.0%) | 4 (1.7%) | - | ||||
| Lobar | - | - | |||||
| Peripheral | 4 (1.7%) | - | |||||
| Perilobular opacities | 32 (7.9%) | 25 (10.7%) | 7 (4.0%) | ||||
| Nodules | 2 (0.5%) | 2 (0.9%) | - | ||||
| Bronchiectasis | 52 (12.8%) | 48 (20.5%) | 4 (2.3%) | ||||
| Central | 1 (0.2%) | 1 (0.4%) | - | ||||
| Peripheral | 44 (10.9%) | 40 (17.1%) | 4 (2.3%) | ||||
| Both | 7 (1.7%) | 7 (3.0%) | - | ||||
| Pattern | OP | 12 (3.0%) | 2 (0.9%) | 10 (5.8%) | |||
| Non-fibrotic NSIP | 103 (25.4%) | 60 (25.6%) | 43 (25.1%) | ||||
| Mixed | 32 (7.9%) | 25 (10.7%) | 7 (4.0%) | ||||
| Residual fibrotic abnormalities | 18 (4.4%) | 11 (4.7%) | 7 (4.0%) | ||||
| Global extension (%), median (IQR) | 30% (20%; 39%) | 30% (20%; 37.5%) | 25% (20%; 50%) | ||||
| Subpleural reticulations | 15 (3.7%) | 10 (4.3%) | 5 (2.9%) | ||||
| Bronchiectasis | 16 (4.0%) | 9 (3.8%) | 7 (4.0%) | ||||
| Central | - | - | - | ||||
| Peripheral | 12 (3.0%) | 5 (2.1%) | 7 (4.0%) | ||||
| Both | 4 (1.0%) | 4 (1.7%) | - | ||||
| Mild | 8 (2.0%) | 4 (1.7%) | 4 (2.3%) | ||||
| Moderate | 8 (2.0%) | 5 (2.1%) | 3 (1.8%) | ||||
| Severe | - | - | - | ||||
| Honeycombing | 2 (0.5%) | 1 (0.4%) | 1 (0.6%) | ||||
| Volume loss | 9 (2.2%) | 5 (2.1%) | 4 (2.3%) | ||||
| Ground glass opacities | 14 (3.5%) | 8 (3.4%) | 6 (3.5%) | ||||
| Pattern | Fibrotic NSIP | 14 (3.5%) | 9 (3.8%) | 5 (2.9%) | |||
| UIP | 1 (0.2%) | 0 (0.0%) | 1 (0.6%) | ||||
| UIP probable | 3 (0.7%) | 2 (0.9%) | 1 (0.6%) | ||||
| Post-ventilatory abnormalities | 10 (2.5%) | 6 (2.6%) | 4 (2.3%) | ||||
| Global extension (%), median (IQR) | 45% (32.5–60%) | 45% (32.5–57.5%) | 60% (35–84%) | ||||
| Subpleural reticulations | 7 (1.7%) | 6 (2.6%) | 1 (0.6%) | ||||
| Bronchiectasis | 9 (2.2%) | 6 (2.6%) | 3 (1.8%) | ||||
| Central | 3 (0.7%) | 3 (1.3%) | - | ||||
| Peripheral | 5 (1.2%) | 2 (0.9%) | 3 (1.8%) | ||||
| Both | 1 (0.2%) | 1 (0.4%) | - | ||||
| Mild | 3 (0.7%) | 1 (0.4%) | 2 (1.2%) | ||||
| Moderate | 5 (1.2%) | 5 (2.1%) | - | ||||
| Severe | 1 (0.2%) | - | 1 (0.6%) | ||||
| Honeycombing | 1 (0.2%) | - | 1 (0.6%) | ||||
| Volume loss | 5 (1.2%) | 3 (1.3%) | 2 (1.2%) | ||||
| Ground glass opacities | 9 (2.2%) | 5 (2.1%) | 4 (2.3%) | ||||
| Cicatricial emphysema | 6 (1.5%) | 4 (1.7%) | 2 (1.2%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besutti, G.; Monelli, F.; Schirò, S.; Milone, F.; Ottone, M.; Spaggiari, L.; Facciolongo, N.; Salvarani, C.; Croci, S.; Pattacini, P.; et al. Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study. Tomography 2022, 8, 1184-1195. https://doi.org/10.3390/tomography8030097
Besutti G, Monelli F, Schirò S, Milone F, Ottone M, Spaggiari L, Facciolongo N, Salvarani C, Croci S, Pattacini P, et al. Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study. Tomography. 2022; 8(3):1184-1195. https://doi.org/10.3390/tomography8030097
Chicago/Turabian StyleBesutti, Giulia, Filippo Monelli, Silvia Schirò, Francesca Milone, Marta Ottone, Lucia Spaggiari, Nicola Facciolongo, Carlo Salvarani, Stefania Croci, Pierpaolo Pattacini, and et al. 2022. "Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study" Tomography 8, no. 3: 1184-1195. https://doi.org/10.3390/tomography8030097
APA StyleBesutti, G., Monelli, F., Schirò, S., Milone, F., Ottone, M., Spaggiari, L., Facciolongo, N., Salvarani, C., Croci, S., Pattacini, P., & Sverzellati, N. (2022). Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study. Tomography, 8(3), 1184-1195. https://doi.org/10.3390/tomography8030097







